- No products in the cart.
Menu
Start typing to see products you are looking for.
Start typing to see products you are looking for.
Browse Categories
-
PPE Apparel
Masks
-
 KN95 Respirator Mask, 4 layers, Box of 50 -LP-KN95-001BOX50
$79.00
KN95 Respirator Mask, 4 layers, Box of 50 -LP-KN95-001BOX50
$79.00
-
 Harley N95 NIOSH Certified Particulate Respirator Mask, Folder Style 20/Box - L-188
$36.00
Harley N95 NIOSH Certified Particulate Respirator Mask, Folder Style 20/Box - L-188
$36.00
-
 Lab Pro Mask KN95 (K-N95) Pack of 50 - LP-MASK2-K95 - 40% OFF
Lab Pro Mask KN95 (K-N95) Pack of 50 - LP-MASK2-K95 - 40% OFF
$125.00$75.00 -
 Lab Pro 3ply Earloop Disposable Mask (Non-Surgical) (Box of 50) - 84% OFF
Lab Pro 3ply Earloop Disposable Mask (Non-Surgical) (Box of 50) - 84% OFF
$57.00$9.00
-
-
Chemicals
-
Wipes
-
Microscopes and Lighting
-
Hand Tools
Hand Tools
-
Gloves
-
Swabs and Applicators
Foam Tipped Swabs
-
ESD & Static Control
ESD & Static Control
-
Lab Equipment
-
Pipettes
-
Furnaces and Ovens
Furnaces and ovens
-
All Products
- Services New
-
Industries Served
- Brands
- Promotions
-
Information
-
Blog
-
All blogs
- Aerospace
- Calibration of Lab Equipment
- Chemicals and Solvents
- Cleanroom and Critical Environment
- Electric Battery Labs
- ESD Safety
- Lab Consumables
- Lab Glassware and Glassware Equipment
- Lab Pro’s Top 5
- Laboratory Equipment
- Laboratory Safety & Lab Efficiency
- Medical Adhesives
- Medical Device Industry
- Microscopes, Lighting & Inspection
- News
- Our Blog
- Pipettes
- PPE and Safety Apparel
- Press Release
- Science Education
- Solar Energy Labs
- Sustainable & Eco-Conscious Lab
- Swabs
- Tweezers and Cutters
- Ultrasonic Cleaning
- VMI for Lab Supplies
-
All blogs
- Contact
- All Categories
- Tians USP 800 Compliant Barrier Gown (30 pack) (Large to X-Large) - 815775-L-XL
Tians USP 800 Compliant Barrier Gown (30 pack) (Large to X-Large) - 815775-L-XL
$108.00
SKU: 815775-L-XL
Warning:
California prop 65
Categories:
Gowns
,USP 800 Gowns
,
Description
Description
The Tians USP 800 Compliant Barrier Gown (815775-L-XL) provides full-body protection for healthcare and cleanroom staff working with hazardous drugs. Specially engineered by Tians, this gown complies with USP <800> standards for handling chemotherapy agents and other hazardous substances.
The gown is constructed from a multi-layer polyethylene and polypropylene fabric, with seams sealed by a proprietary co-polymer tape for complete barrier protection. Features include tunnelized elastic wrists, thumb loops, Velcro neck closure, and extra-long coated waist ties for secure fastening at the front or back.
Each gown is vacuum-sealed, inspected for workmanship, and labeled for USP 800 compliance. Designed for safety and durability, these gowns exceed FDA, NIOSH, OSHA, ASHP, and ONS guidelines.
Key Features
- USP 800 compliant: for hazardous drug handling.
- Multi-layer polyethylene/polypropylene: barrier fabric.
- Taped seams tested: for liquid and hazardous drug resistance.
- Tunnelized elastic wrists and thumb loops: to secure sleeves.
- Velcro neck loop closure: for adjustable fit.
- Extra-long coated waist ties: for versatile closure.
- Blue gown with blue taped seams: for easy identification.
- Individually inspected: vacuum-sealed packaging.
Applications
- Hazardous drug preparation and handling.
- Chemotherapy drug protection.
- ISO 6 cleanroom and controlled environments.
- Healthcare facilities, pharmacies, and compounding labs.
- Worker and patient safety where bloodborne pathogens or splashes are a concern.
Benefits
- Ensures compliance: with USP <800> standards.
- Protects staff: from bloodborne pathogens and hazardous liquids.
- Strong, durable design: with tested seam and fabric barriers.
- Comfortable fit: with secure wrist, thumb, and neck closures.
- Backed by trusted quality: of Tians.
Product Specs
| ISO Class | 6 |
| Fabric Weight | 60 gsm |
| Grab Strength | MD: 20.3 lbs, XD: 14.5 lbs (ASTM D751) |
| Ball Burst Strength | 20.3 lbs (ASTM D3787) |
| Puncture/Tear Resistance | MD: 4.9 lbs, XD: 5.8 lbs (ASTM D2582) |
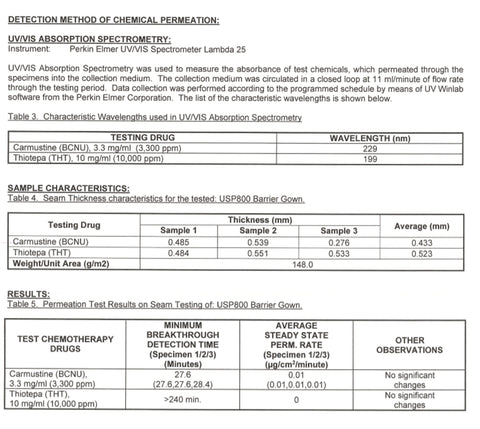
| Permeation | Tested against 12 hazardous drugs, 27–240+ minutes |
| Bloodborne Pathogen Resistance | Pass (ASTM F1671) |
| Flammability | Class 1 (CPSC 1610) |
| Color | Blue with blue taped seams |
| Packaging | 10 gowns per inner bag, 3 bags per master bag (30 gowns per case) |
Categories
- Cleanroom Apparel
- USP 800 Compliant PPE
- Hazardous Drug Protection
- Tians Products
- Gowns & Protective Wear
FAQs
How should L–XL fit for safe movement?
The gown should allow full reach without pulling at the shoulders and should wrap at the waist without opening gaps. Thumb loops must stay engaged during reach and lift.
Can I secure ties in front for quicker doffing?
Yes. Extra-long coated ties can fasten front or back. For chemo workflows, many sites prefer front-tie for faster emergency doffing.
Do I still need double chemotherapy gloves?
Yes. USP <800> requires ASTM D6978-tested chemo gloves worn double, with the outer glove covering the gown cuff.
Are these gowns suitable for sterile compounding?
They provide hazardous drug barrier protection. For sterile compounding, add sterile outer layers (e.g., sterile sleeves or sterile outer gloves) per your aseptic SOP.
How are damaged gowns handled mid-task?
Stop work, doff carefully to avoid skin contact, dispose of hazardous waste, perform hand hygiene, and don a new gown and gloves before resuming.

























































